how to find oh concentration from ph
pH Calculator
Calculate pH using the chemical concentration or mass and volume.
What is pH?
pH is a measure of the acidity or alkalinity of a chemical solution. It's a numerical value expressing how acidic or basic a liquid solution is.
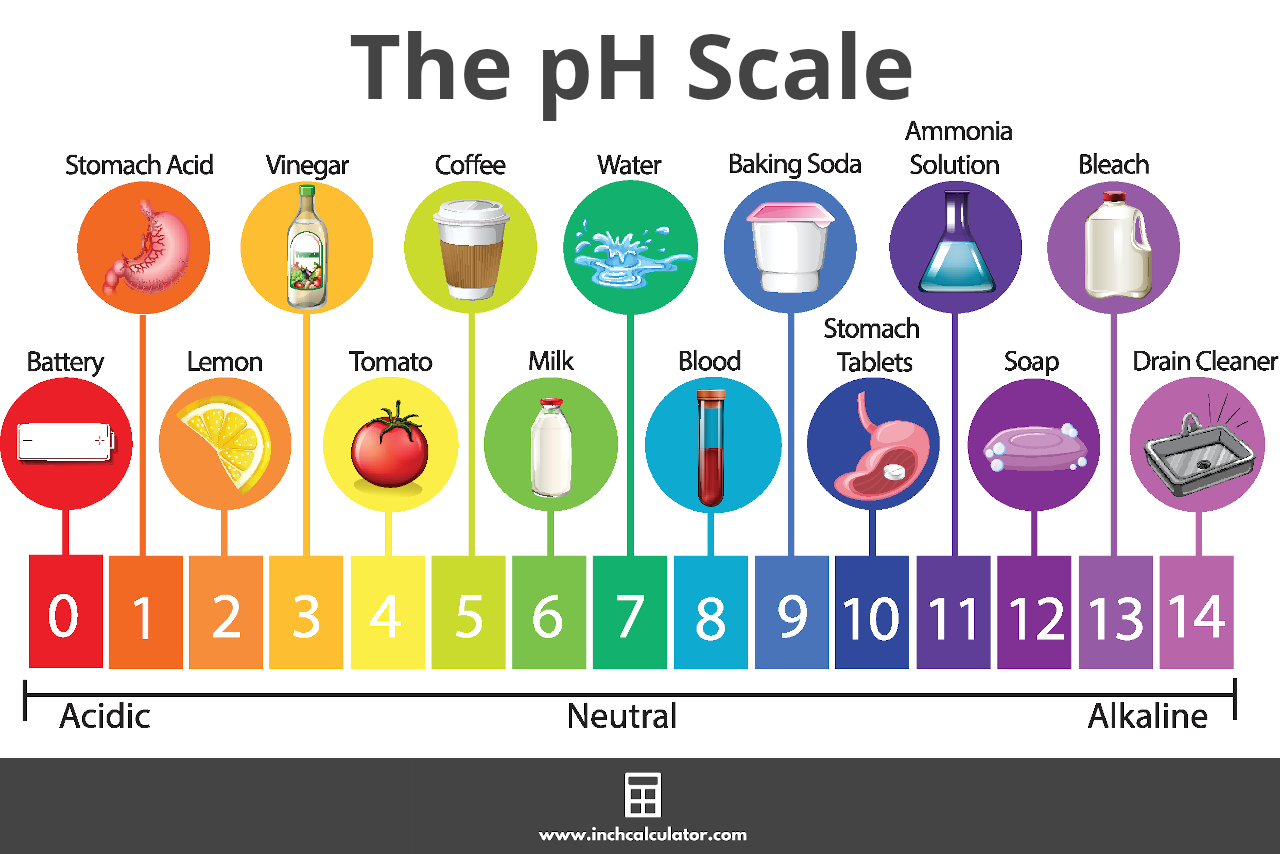
The pH Scale
pH is measured on a scale ranging from 0 to 14. A pH of 7 is considered neutral.
Values lower than 7 are acidic, and the lower the pH value, the more acidic the solution is. Values higher than 7 are basic, with higher values being more alkaline.[1]
How to Calculate pH
You can calculate pH if you know the molar concentration of hydrogen ions in the solution. Since pH is defined as the negative logarithm of the hydrogen ion concentration, you can calculate it using a formula.[2]
pH Formula
The formula to find pH is:
pH = −log[H+]
Thus, the pH of a solution is equal to the negative base-10 logarithm of the hydrogen ion concentration [H+].
For example, let's calculate the pH of a solution with a hydrogen ion concentration of 0.0000463.
pH = -log(0.0000463)
pH = -(-4.334)
pH = 4.334
pOH to pH
pOH is a measure of the concentration of hydroxide ions (OH–), and the sum of pH and pOH is always equal to 14.
pH + pOH = 14
Thus, you can calculate pH if you know this value using the following formula:
pH = 14 – pOH
As you can see from these formulas, the higher the concentration of hydrogen ions, the lower the pH will be, and the more acidic the solution will be. On the other hand, the higher the concentration of hydroxide ions, the higher the pH will be, and the more basic or alkaline the solution will be.
How to Calculate H+
If you know the pH of a solution, you can find the hydrogen concentration by reversing the sides in the formula above.
pH to H+ Formula
So, the pH to [H+] formula is:
[H+] = 10-pH
The hydrogen ion concentration [H+] is equal to 10 to the power of the negative pH value.
How to Find pH from Molarity
The molarity of a solution is equal to the hydrogen concentration [H+] in moles per liter. Given this, you can find the pH of a solution using the molarity with the formula above.
pH = −log[H+]
pH is equal to the negative base-10 logarithm of the molarity of the solution, which is the hydrogen ion concentration [H+].
References
- United States Geological Survey, pH and Water, https://www.usgs.gov/special-topic/water-science-school/science/ph-and-water
- Determining and Calculating pH, 2020, August 15, https://chem.libretexts.org/@go/page/1292
how to find oh concentration from ph
Source: https://www.inchcalculator.com/ph-calculator/
Posted by: esquivelsest1967.blogspot.com

0 Response to "how to find oh concentration from ph"
Post a Comment